
Cooling and freezing are methods that have been known for centuries to extend the shelf life of food. A lower temperature slows down the chemical reactions in the product and reduces microbiological growth, but it also changes its physical properties. So how can you do that efficiently and effectively, while maintaining product quality? An overview.
When a product is chilled or frozen, its temperature decreases because energy (heat) is extracted from it. This energy is transported from high to low temperature by conducting and convection. Conduction takes place primarily within the product, while convection and/or conduction takes place primarily on the outside of the product. The heat removing capacity by conduction also depends on the composition of the product (thermal conductivity): ice has a higher thermal conductivity than water.
Each product, depending on its composition, has a specific heat which is expressed in kJ/(kg °C). This represents the amount of energy that must be extracted to make 1 kg of that product drop or rise by 1°C in temperature. Each product also has a certain point (or a temperature range) at which the water present changes into ice; the solid form of water. The quantity of heat for solidification of melting is called latent heat. The amount of kJ that is required to convert 1 kg of the product from liquid to solid. Pure water has a solidification point at 0 °C. Most water based products start freezing just below 0 °C and the crystallisation of the water content in de product gradually increases while lowering the temperature. So the water in food does not freeze at just a single moment, but over a temperature range. Besides, in many food products a part of unfrozen water remains (see table).
| Temperature | -5°C
|
-10°C | -20°C | -30°C | -65°C |
| Percentage of ice | 75% | 82% | 86% | 87% | 88% |
Table: Indication of the amount of ice as a percentage of the total amount of water depending on the temperature of the meat
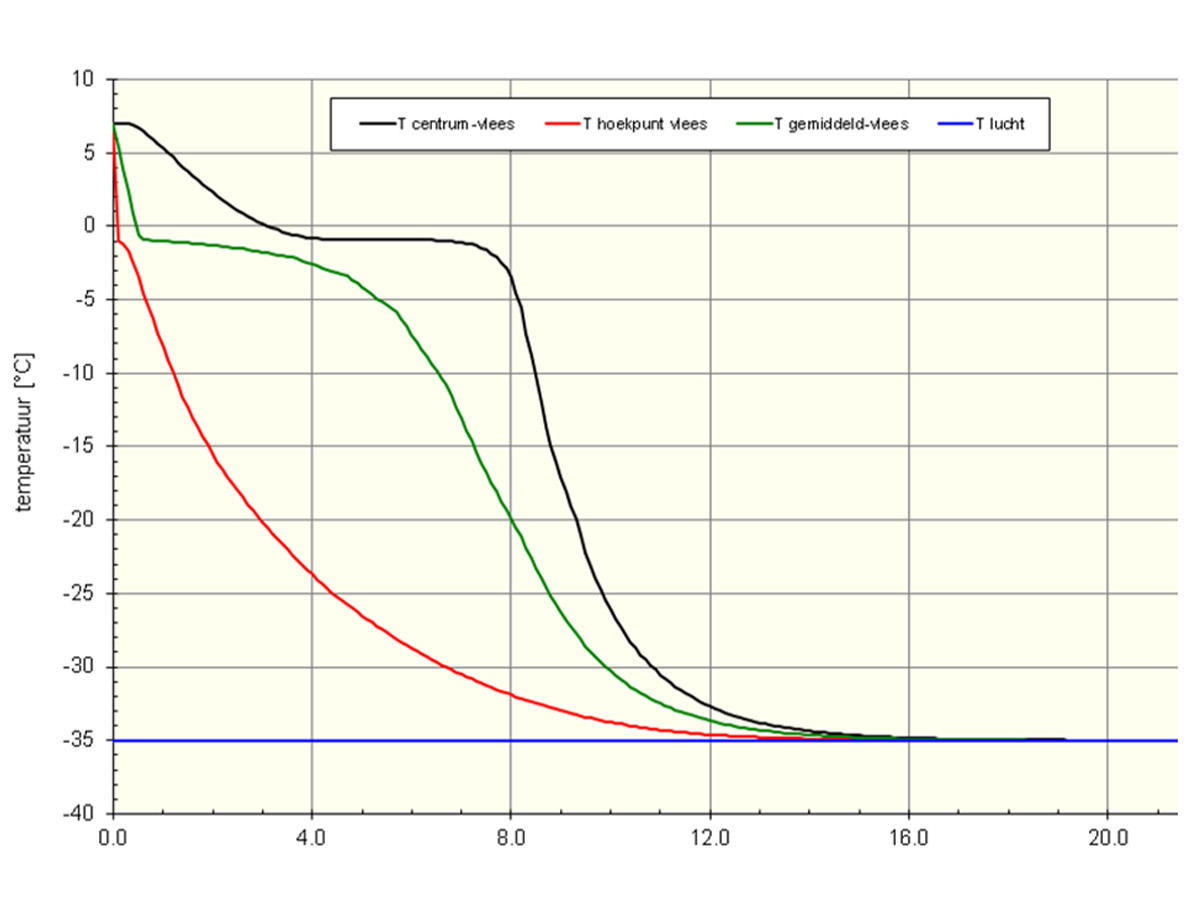
Figure: Temperature development during freezing. Freezing lean meat in cardboard box 60 x 30 x 10 cm; air conditions -35 °C and 5 m/s
The higher the air speed along the surface of the products, the more energy can be removed per unit of time. For cooling and freezing, a turbulent high speed air flow, cools down faster by a factor of 10 than compared with a low air speed flow. The same applies to water. The more turbulent water flows past the product to be cooled, the better the heat transfer (more effective). An additional way to increase the cooling speeds is to spray more water on the surface of the product. This water will evaporate. To evaporate moisture from a surface, energy is also required and this is the additional cooling effect. Cooling with a liquid is more efficient than cooling with air.
The thermal conductivity in a product strongly depends on the composition (density) of the product, the moisture content and the temperature difference. A product with a low density and/or low moisture content has a low thermal conductivity. The most important parameter for cooling is the temperature difference between the product and its environment. The bigger this temperature difference is, the faster the energy can be extracted from the product. When the product approaches the temperature of its surroundings, the temperature gradient becomes lower, resulting in very low thermal conductivity. Effective cooling is then no longer an issue.
When freezing products, take into account the latent heat has to be removed as well for freezing the water; this is a substantial part of the total amount of cooling capacity! We have illustrated this in the figure. It is clearly visible that the decrease in temperature above the solidification point is quicker than below the solidification point. The temperature difference with the air flow gradually reduces and the transition from liquid to solid takes a considerable amount of capacity. This is shown by the difference between the vertex (red line) and the centre of the block (black line).

When freezing especially very moist products, crystallisation first takes place in the extracellular space (space between the cells) and when temperature drops it starts in the intracellular space (in the cell itself). The effect of freezing on the structure of muscle or cell plasma therefore depends on the speed of this process. Slow freezing (freezing rate below 1 cm/hour) produces large ice crystals, mainly in the extracellular spaces. Moisture will be transported from the cells to space between the cells. These large ice crystals also damage the cell walls. Damaged cells give more drip loss during defrosting. At high freezing rates (freezing rate greater than 2 cm/h), more small ice crystals are formed both outside and inside the muscle cells (meat) or plasma in the vacuole (vegetables, fruit). The water in the cells can now be better re-absorbed by the product during defrosting: the binding of water is better retained. This results, for example, in less drip loss during defrosting of meat products.
Fast cooling or freezing requires a good heat transfer and a sufficiently low temperature. The most efficient way to achieve good heat transfer is cooling/freezing via direct contact with a refrigerated contact area. Partly because of this, plate freezers are much more efficient than air freezing tunnels. Circulating the air at high speed requires a relatively large amount of additional electrical energy for the fans and the heat transmission rate is still much lower than cooling by direct contact. Improving the heat transfer rate enables an increase of the evaporation temperature, for the same cooling/freezing speed. Each degree raise of the evaporator temperature increases the efficiency of the refrigeration installation by 3 to 4%. Note that a large piece of meat can never be frozen as quickly as small pieces, not even with low temperatures and high heat transfer capacity at the surface of the product. This is soly because the fact that heat must be removed from the core of the product. That is a slow process, which is hard to speed up, other than making small pieces! So freezing small pieces is always better!
In short: cooling and freezing are processes that can improve the shelf life of food products, and help to better process them. The method and speed of this process determine the structure and quality of the product after defrosting.
Mainphoto: ©bodnar.photo/Shutterstock.com, photo meat: Sungsu Han/Shutterstock.com
Source: Vakblad Voedingsindustrie 2022